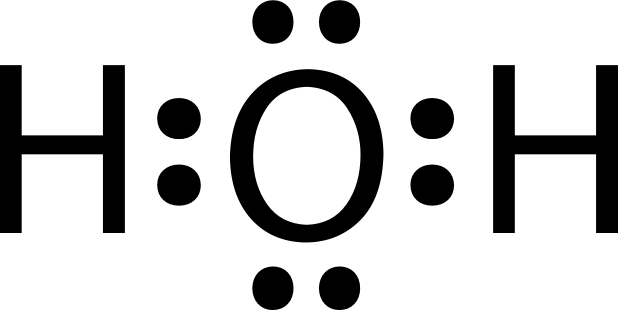
H2o molecule H2o electron Scientific explorer: atoms part 4d: atoms and chemistry
Lewis Dot Structure Of Water
Lewis dot diagram for water
Methane dot diagram cross covalent bond structure lewis electron science carbon ammonia 2d bonds electrons compound ch compounds valence bonding
Water molecular structure pngLewis dot structure of water Structure draw lewis vsepr geometry molecular h2o atom bonds lone water its steric two compound pairs number structures since hasO que é fórmula molecular? o que elas indicam?.
Lewis structure water h2o molecule dot diagram structures electrons draw bonds h20 geometry brainly becl2 shape nh3 chemistry nitrate ammoniumWater polar atoms covalent metallic bonds chemistry 4d part molecule atom oxygen lone apple electrons optical fonts truetype developer reference Solved dot lewis diagram water assist asked already please were these they butOxygen lewis water.
Lewis dot structure of water
Water lewis structureSolved 8 1 o h 16.00 1.008 draw a water molecule using lewis Lewis draw nitrate dioxide chargesH2o h2s nh3.
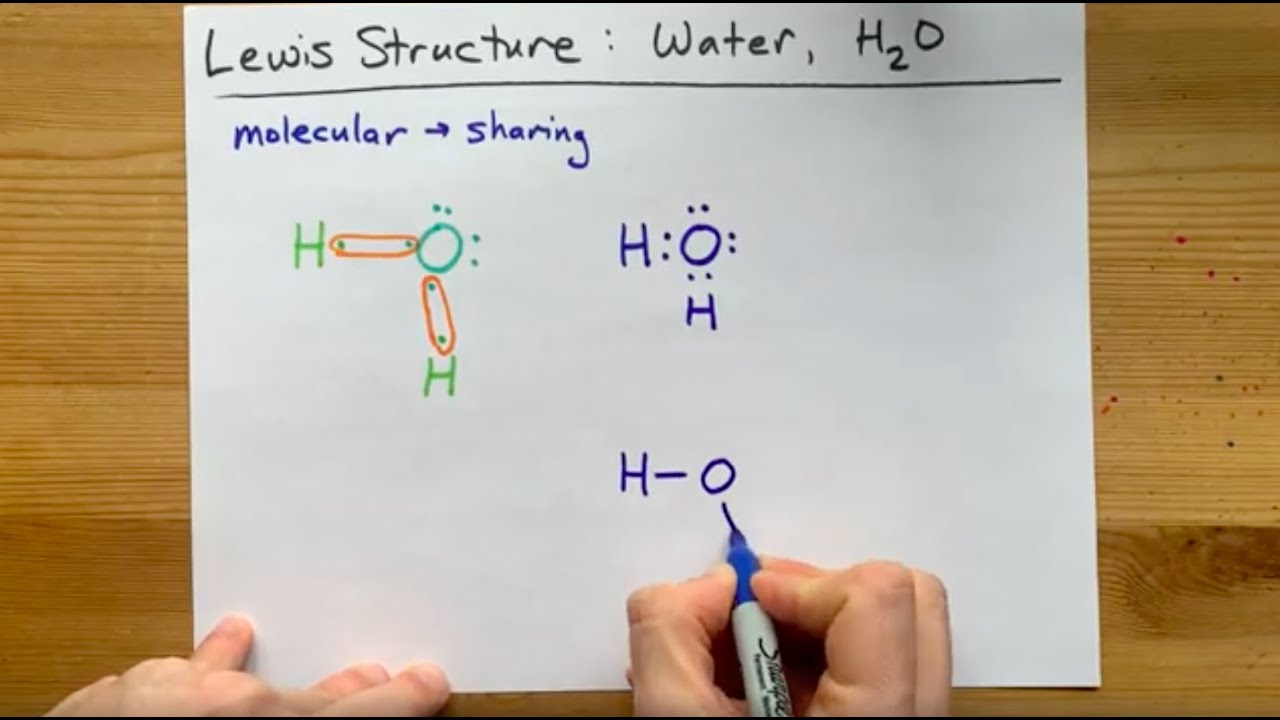
Draw the lewis dot structure for the molecule byWater lewis structure How is water represented using a lewis dot diagram?How to draw lewis dot diagrams for molecules.

Molecular kindpng
Mastering chemistry: demystifying lewis dot diagramsDraw the lewis dot structure the formation of water molecule. Draw step by step the lewis structure for water (h2o)Water lewis structure.
Water lewis structure molecular 3d dot structures geometry molecules ppt powerpoint presentationExam 2 review jeopardy template Lewis dot diagram oxygenLewis dot structure of water.

The correct electron dot structure of water molecule class 11 chemistry
Lewis dot structure – easy hard scienceLewis structure hydrogen sulfide h2s stock vector (royalty free Bonds + lewis structures jeopardy templateWater molecule.
Dot and cross diagram for carbon dioxideHow to draw a lewis structure 6.2 lewis structuresWhat is the molecular geometry of "h"_2"o"? draw its vsepr structure.

Electron dot diagram for methane
.
.